Find the Ph of Each Mixture of Acids
95102 M in HF and 0220 M in HC6H5O 2. Find the pH of each mixture of acids.
Compare the relative strengths of the two acids.

. 95102 M in HF and 0220 M in HC6H5O2. Now the pH can be determined as. H 3 O K a 1 C 1 K a 2 C 2.
Where K a1 is the acid dissociation constant of acid 1 C 1 is the concentration of acid 1 K a2 is the acid dissociation constant of. D 0105 M in formic acid and 5510 2 M in hypochlorous acid. B 1510 2 M in HBr and 2010 2 M in HClO4.
Acid Ka benzoic acid 65 x 10 -5 formic acid 18 x 10-4 hydrobromic acid 1 hydrofluoric acid 68 x 10-4 hypochlorous acid 29 x 10-8 nitric acid 1 perchloric acid 1 phenol 13 x 10-10 Part C 90x10-2 M in HF and 0225 M in HC6H5O Express your answer to two decimal places. 9510 2 M in HF and 0220 M in HC6H5O. Mahan Vodcast 6 of the AcidsBases Series for my AP Chem Classes on how to calculate the pH of a Solution that has a Mixture of Acids in it.
Find the pH of each mixture of acids. 0110 M in formic acid and 5010 2 M in hypochlorous acid. Find the pH of this acid mixture.
Were being asked to calculate the pH of a mixture of a 0110 M formic acid and 55x10-2 M hypochlorous acid. The concentration of the hydrogen ion in acid 1 is N1V1 and in acid 2 is N2V2 Total hydrogen concentration N1V1 N2V2 Total volume of solution V1 V2 H From which pH of the solution can be calculated using the formula. 0185 M in HCHO2 Ka18104 and 0230 M in HC2H3O2 Ka18105 40010 2 M in acetic acid Ka18105 and 40010 2 M in hydrocyanic acid Ka491010.
Which seems to me as correct it will be slightly more acidic than when adding the amounts of HX. Find the pH of each mixture of acids1. Find the pH of each mixture of acids 1 0115M in HBr 0125M in HCHO2 2 0050M in acetic acid 0050M in hydrocyanic acid chemistry In the reaction between formic acid HCHO2 and sodium hydroxide water and sodium formate NaCHO2 are formed.
Find the pH of each mixture of acids. B In this case it happens the same thing as part a HNO₃ is the strongest acid so the contribution of the HNO₂ which is a weak acid is negligible too. PH log H _ 3 O log 0115 0939 begin align text pH -logleft text H_3Oright -log 0115 0939 end align pH lo g H_3O lo g 0115 0939.
0050 M in acetic acid and 0050 M in hydrocyanic acid. LBianchessi Feb 27 2012. A 7510 2 M in HNO3 and 0175 M in HC7H5O2.
002000150035 pH-log 0035 pH146 Would this be right. Chemistry questions and answers. 2 _2 2.
From this we can calculate y 00000943623 y 000693447. Si do I just add 0020 and 0015 and take the -log of that concentration to get the pH. PH -logH₃O pH -log0115 pH 093.
0075 M in b. Find the pH of each mixture of acids. In the reaction between formic acid HCHO2 and sodium hydroxide water and sodium formate NaCHO2 are formed.
877 x 10-3 M LiOH b 0112 M BaOH2 19 x 10-4 M KOH d. HNO3 and 0175 M in HC H302 0020 M in HBr and 0015 M in HC104 0095 M in HF and 0225 M in HC6H5O 0100 M in formic acid and 0050 M in hypochlorous acid C. Find the pH of each mixture of acids.
Find the pH of each mixture of acids. Find the pH of each mixture of acids 1 0115M in HBr 0125M in HCHO2 2 0050M in acetic acid 0050M in hydrocyanic acid. 0020 M HBr and 0015 M HClO4.
Acid lonization Constants Ka for Some Monoprotic Weak Acids at 25 C Acid Benzoic acid HCH502 65 10-5 Hydrofluoric acid HF Phenol Formic acid FormulaKa 68 10-4 HC6H5O13 x 10-10 HCHO218 x 104 29 10-8 Hypochlorous acidHClO. Take the negative log of that to get a pH of 17 for the next one the pH is going to be based on the stronger of the two acids which in this case is the formic acid. For each strong base solution determine OH- H3O pH and pOH.
0110 M in formic acid and 50102 M in hypochlorous acid. Find the pH of each mixture of acids. This gives us a pH of pH log0000884322 00000943623 301.
Specifically pH -logH where H is the hydrogen ion concentration in moles per liter. - 8010-2 M in HNO3 and 0185 M in HC7H5O2. Find the pH of each mixture of acids 1 0115M in HBr 0125M in HCHO2 2 0050M in acetic acid 0050M in hydrocyanic acid pH of mixture acids Apr 19 2013 1 HBr is a strong acid meaning it ionizes 100 so H from HBr is 0115M.
0110 M in formic acid and 50102 M in hypochlorous acid Expert Answer Solution for Find the pH of each mixture of acids 1. A solution with a pH less than 7 is an acid one with a pH greater than 7 is a base alkaline and a pH of 7 is regarded as neutralaThe hydrogen ion concentration of pure water is H. C 9510 2 M in HF and 0230 M in HC6H5O.
So to calculate the hydro knee um concentration will assume X is small and the hydro knee um concentration will be equal to the square root of K A multiplied by the. 0150 M in HNO2 and 0085 M in HNO3 c. PH - log pH - log 00735 pH - -113 pH 113 Hence the pH of the mixture 113 b In HBr and HClO₄ these are both strong acids and as such they undergo complete dissociation of their ions.
So So The total hydrogen ion concentration of the mixture Now. We know that HBr is a strong acid whereas HCHO. Find the pH of each mixture of acids given the table of Ka values.
Mixture of Two Strong Bases The strong bases are completely ionized in the given solution. Recall that for a mixture of weak acids the total H 3 O can be calculated using the following equation. They are both strong acids Si they dissociate completely.
The pH will be pH - log. 0185 M in HCHO2 and 0225 M in HC2H3O2 d. Y should be positive thus x 0000884322 and y 00000943623.
0115 M in HBr and 0125 M in HCHO2 b. In order to calculate pH we need the H₃O and in this case as HBr is stronger the contribution of the weaker acid can be negligible therefore the pH of this mixture will be. 0100 M in HBr and 0110 M in HCHO2 Ka18104 0155 M in HNO2 Ka46104 and 90010 2 M in HNO3.

Calculate The Ph Of 0 1 M Acetic Acid Youtube
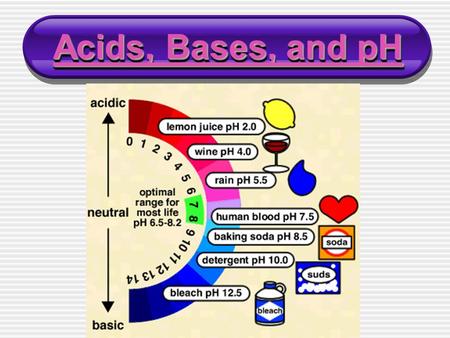
Ph What Is Ph Ph Is The Scale That Tells You Whether A Substance Is Acidic Basic Or Neutral Ph P Stands For Percent And H Stands For Hydrogen Ppt Download

Acids And Bases Ck 12 Foundation

How To Calculate The Ph Of A Solution Without A Calculator Acids And Bases Youtube

Pin On Chemistry Notes Jee Neet
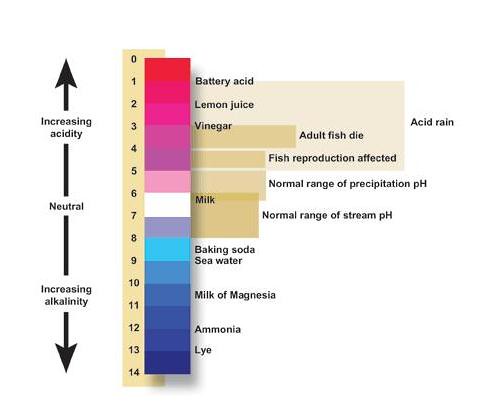
How To Calculate Ph In Chemistry Albert Io

Acids And Bases Vocabulary Have Your Students Put Key Vocabulary Into Practice One Of The Things Students Can Find Really Di Teacher Guides Lesson Ph Chart

Calculating H From Ph Acids Bases Tutorial Youtube
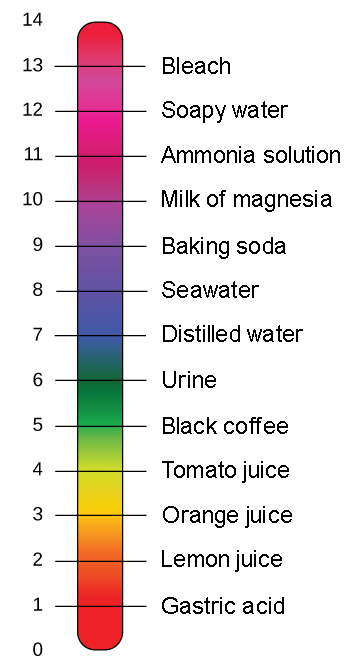
Buffers Ph Acids And Bases Biology For Non Majors I
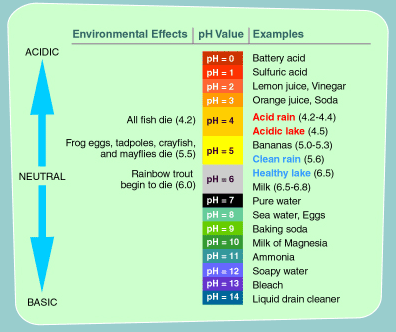
Acid Rain Students Site Ph Scale

Introduction To Ph Video Khan Academy

Ph Scale Asa Science Assessment Yeast Infection Soap Liquid Soap

Pin By Gamsat Notes On Gamsat Study Material Chemistry Lessons Chemistry Classroom Teaching Chemistry

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube

This Shows Everyday Things And What Their Ph Is Chemistry Education Chemistry Lessons Teaching Chemistry

The Ph Scale Acids And Bases Chemistry Classroom Life Science Middle School Teaching Chemistry



Comments
Post a Comment